Research Objectives (background)
Musculoskeletal disorders such as osteoarthritis of the knee (OA) not only decrease activities of daily living (ADL) but also negatively impact quality of life (QOL). OA is an intractable degenerative disease of the joint that is slow progressing and widely prevalent. To date, no fundamental treatment exists, and with a global aging population, the increasing number of OA patients is a serious concern, making a fundamental treatment for OA imperative.
Regenerative medicine for articular cartilage has been implemented domestically on one hand, but current treatments fail to address cartilage defects associated with degenerative diseases such as OA. Our research focuses on treating such difficult to treat cartilage defects with the regeneration of hyaline cartilage, which better supports the mechanistic function of normal joints.
Prior to Clinical Studies
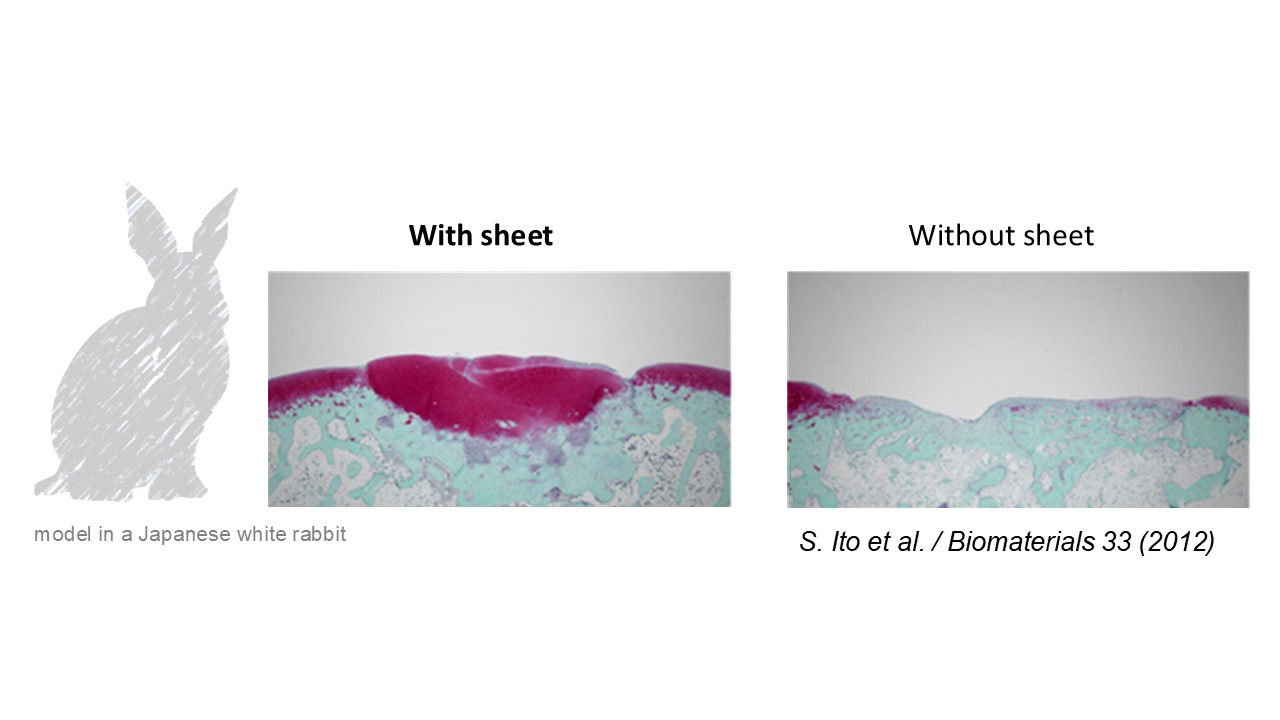
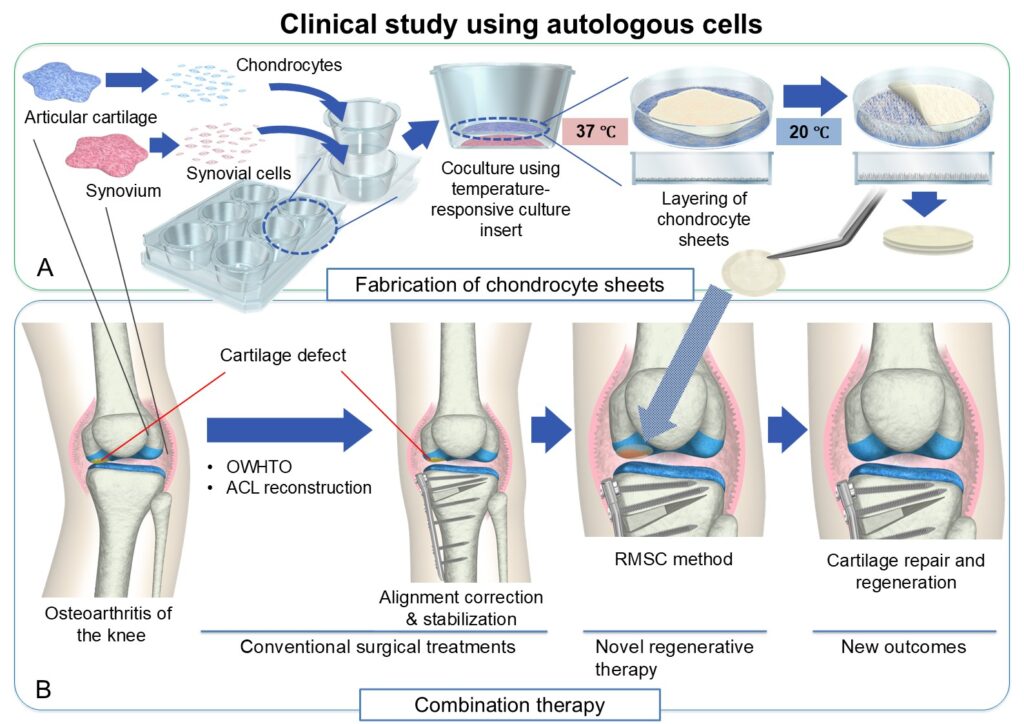
We have conducted basic research under the concept that as long as the surface layer of articular cartilage is regenerated properly, the underlying and deep layers can self-regenerate through intrinsic regenerative functions. “Cell sheet” technology developed by Emeritus Professor Teruo Okano at the Tokyo Women’s University allows the collection of cells and surrounding extracellular matrix from the culture dish, noninvasively and without enzymatic treatment, in the form of a thin sheet, using a specially coated temperature-responsive culture dish. We have confirmed the efficacy of chondrocyte sheets fabricated on such temperature-responsive culture dishes in various animal experiments, and specifically, the treatment effects of such sheets on both partial-thickness defects (those that do not reach the subchondral bone) and full-thickness defects (those that reach the subchondral bone) are worth special mention in terms of their uniqueness in regenerative medicine.
However, in order to make this treatment available clinically, rigorous safety evaluations were necessary. We had hoped to make this treatment available rapidly, but more than three years were spent to meet regulatory guidelines of the Ministry of Health, Labour and Welfare of Japan, and finally in 2012, we began a clinical study for the transplantation of chondrocyte sheets fabricated from the patients’ own cells (transplantation of autologous chondrocyte sheets). We confirmed the safety of this treatment method and the improvement in clinical scores, and verified the regeneration of hyaline cartilage in all eight patients entered into the study. And to further expand the clinical prospects of chondrocyte sheets and to transfer the technology to industry, in 2017, we began our second clinical study using allogeneic chondrocyte sheets fabricated from cells collected from other individuals.
Future Prospects
As of April 2025, all patients in the clinical study for the transplantation of autologous chondrocyte sheets have reported favorable progress in their recovery at more than 11 years post-surgery, and we performed this treatment as Advanced Medical Care B. At the same time, 10 patients who have been treated through the transplantation of allogeneic chondrocyte sheets have reported favorable progress in their recovery at more than 5 years post-surgery, and we are in preparation to offer this treatment as the clinical study for the joint treatment by next-generation allogeneic chondrocyte sheets.
Our focus now is to further determine the potential of chondrocyte sheets for the treatment of cartilage defects associated with OA through the clinical trial and to make this treatment available clinically. Furthermore, we continue to work in hopes that our daily research lead to a fundamental and overall treatment for OA, so as to contribute to the increase in patient ADL and QOL through the preservation of one’s own joints.